Viral vaccines and viral vectors used in biotherapeutic applications carry the risk of microbial contamination, which must be addressed.
By Anissa Boumlic, Martin Wisher, Damon Asher, Priyabrata Pattnaik
Vaccines, including viral vaccines, are a crucial invention in human history and continue to improve lives through the prevention, control, and eradication of infectious disease. Viral vaccines rely on antigenic properties of a virus or virus-like particle (VLP) to trigger an immune response against an incipient viral infection. Because of the risks associated with live and inactivated viruses, namely potential attenuation reversal or failure of inactivation, recombinant viruses have emerged in the role of either vaccines or vectors in gene and immunotherapies. However, because biological materials—cell substrates and often animal-derived products—are used in their manufacture, viral vaccines and vectors are at risk of contamination from micro-organisms, such as adventitious viruses.
In the past, a few medicines produced from biological materials, such as blood products or vaccines, were found to be contaminated with viruses. Unscreened human or animal-derived products, such as bovine serum, are now known as potential sources of bovine virus contamination (1).
Since then, better safety measures and the use of established and characterized cell lines have improved safety in biologicals. To date, no infectious virus has been transmitted to a patient by a cell-line-derived biopharmaceutical. Extraneous vesiviruses, however, have recently appeared in bioreactors (2–5), and porcine circovirus type 1 (PCV-1) contamination of oral rotavirus vaccines was first reported by a metagenomics analysis (6). PCVs entered vaccine processes via porcine trypsin, a common cell-culture reagent. In 2014, using next-generation sequencing (NGS), FDA’s Center for Biologics Evaluation and Research (CBER) retrovirus laboratory identified a novel rhabdovirus in Spodoptera frugiperda type 9 (Sf9) cells (7). Sf9 cells are a common substrate for biological products such as VLPs.
These contamination events highlight the limitations of current technologies; more vigilance is needed. Consequences of vaccine or vectorviral contamination are significant, and manufacturers may be forced to recall lots or shut down facilities for decontamination, which can hurt a company’s reputation. Moreover, such events could affect the broader perception of vaccine and viral vector safety.
This article outlines the risks and challenges associated with managing viral safety in virus vaccines and vectors for gene therapy, and highlights a holistic risk-mitigation approach of testing and clearance methodologies to help prevent contamination events.
Viral safety strategy and regulatory
Virus safety of viral vaccines and vectors ensures that: the vaccine product is free of unintended viruses; any residual pathogenicity of a live agent is within acceptable limits for safe use; and inactivated agents are indeed completely inactivated (8). Regulatory guidance documents (9–11) suggest that the risk of adventitious agent contamination should be assessed and mitigated through a tripod strategy (Figure 1):
• Preventing entry of contamination into production processes by ensuring the quality of raw materials
• Detecting contaminants by characterization of cell banks/virus seed stocks and by testing process intermediates
• Eliminating contaminants by incorporating virus inactivation/removal steps into the vaccine purification process (challenging for whole live virus vaccines but practical for inactivated, subunit, and recombinant vaccines).
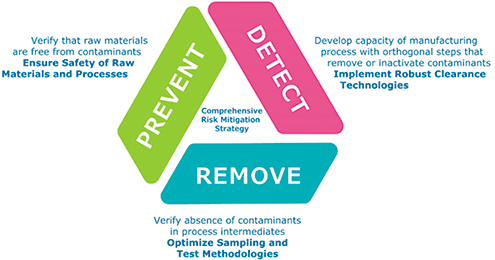
Figure 1. Tripod strategy for assessment and mitigation of the risk of adventitious agent contamination in viral vaccines and vectors.
Prevent virus introduction by testing raw materials
Ensuring the quality of raw materials used in vaccine and vector production is the first step in preventing viral contamination. Animal-derived components such as bovine serum and porcine trypsin should be screened for bovine and porcine viruses. Regulatory guidelines exist for the selection, qualification, and testing of these raw materials and indicate that not only known, but also emerging viruses should be sought (12–16). There are also specific guidelines for the usage of bovine serum or trypsin in the manufacture of biologics (17, 18).
New technologies for cell culture or raw-material treatment create barriers to viruses and mitigate bioreactor contamination risk. These methods primarily target the cell-culture medium before its transfer to the bioreactor as well as raw materials of animal or human origin. Options include high temperature/short time (HTST), C spectrum ultraviolet light (UV-C), gamma irradiation, and nano- or virus filtration.
HTST is commonly used in the food and beverage industry for high-volume processing. A liquid is heated and held at 102 °C for at least 10 seconds, then cooled to 37 °C before being sent to the bioreactors. Virus inactivation efficiency has been demonstrated on several viruses; for example, a 10,000-fold reduction in foot-and-mouth disease virus (FMDV) was observed in HTST-treated milk (19). However, this method might be less effective where high levels of contamination are present (20). This technique has been applied to treat raw materials such as cell-culture media (21) and glucose (22). The impact of HTST on the properties of treated raw material and the performance of cell-culture processes needs to be assessed.
UV-C irradiation has been used in the food, plasma, and biotech industries (for packaging and surface sterilization) and is effective against various biological contaminants, including bacteria and viruses (23–25). Limitation of this method is the flow rate of the fluid, which needs to be modulated for optimal results (21).
Gamma irradiation—ionizing radiation from a radioactive Cobalt 60 source—breaks bonds in nucleic acids and proteins. This method is widely used in the biopharmaceutical industry to treat bovine serum or single-use components. Certain viruses, however, have shown resistance to gamma rays (26).
Nanofiltration or viral filtration is a separation method based on size exclusion and the usage of membranes or fiber-based filters. The biotechnology industry utilizes this technology to ensure viral clearance in downstream processing. Currently, optimized filters are considered for the filtration of thermo-sensitive raw materials and cell-culture media (27).
Because of potential impact on cell-culture performance, downstream processing, and product quality, the treatment method for the prevention of virus contamination should be chosen with care. Figure 2 summarizes current technologies for cell culture medium treatment—and their implications.
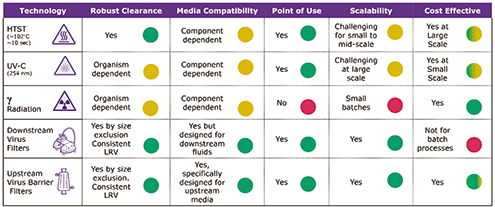
Figure 2. Current barrier technologies for cell-culture medium treatment, with considerations.
Detect viruses in process intermediates
The second step in ensuring viral safety for vaccines and vectors is to test for viruses that could be present in the initial process, beginning with the cell bank. Testing for virus contamination is part of cell-bank characterization. The master cell bank (MCB), the starting material for the entire production process, requires a one-time, full characterization of microbial and viral contaminants. The working cell bank (WCB) requires less testing on early passages, but more on subsequent ones. And end-of-production cells (EOPC) or cells at the limit (CAL) of in-vitrocell age used for production—which represent the worst case for amplification of contaminants—require full, one-time characterization at production scale. This testing is summarized in Figure 3.
MCBs should be tested for identity (phenotypic and genotypic, if recombinant) and purity. While FDA, the European Medicines Agency, and the World Health Organization guidelines differ, testing must demonstrate the absence of bacterial, fungal, and viral contamination. A broad array of in-vitroand in-vivoassays may detect extraneous viruses. In a US National Institutes of Health study, lead investigator Rebecca Sheets, PhD, systematically characterized the breadth and sensitivity of routine in-vitroand in-vivoassays for inapparent viruses (28). These data should aid regulators and manufacturers in decision-making and serve as a baseline for comparison of new methods. Cell lines must also be tested for species-specific viruses, as appropriate, using antibody tests or polymerase chain reaction (PCR) panels.
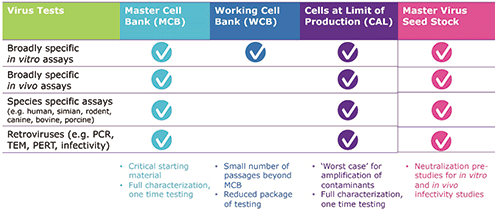
Figure 3. Test strategy for ensuring cell banks and master virus seed stocks begin and remain virus-free.
Beyond these tests, other techniques for the detection of retroviruses may be implemented. Retrovirus particles can be detected using transmission electron microscopy (TEM). Reverse transcriptase enzyme activity within the retrovirus protein core can be detected using PCR-based reverse transcriptase (PBRT) assays, also called product enhanced reverse transcriptase (PERT) assays. Speciesspecific retrovirus screening also exists.
In addition to screening the cell lines, master virus seed stock (MVSS) must also be screened fully to detect adventitious bacteria, fungi, mycoplasma, and extraneous viruses, while taking into consideration the origin and isolation of the virus stock. Neutralizing antiserum is required for infectivity assays to specifically inactivate the master virus. Again, testing must include species-specific assays as well as testing for retroviruses (Figure 3).
Recently, NGS has been applied for viral detection in biologicals. This method allows simultaneous sequencing of millions of DNA or RNA fragments and requires no prior knowledge of potential virus contaminants (29).
Remove or inactivate viral contaminants downstream
Regulatory bodies require that the purification process for a biological pharmaceutical removes any nonproduct virus. These “viral clearance” or “viral removal” steps usually entail inactivation, chromatography, and/or virus filtration.
The fact that viral vaccines and vectors are actual viruses limits the application of removal and inactivation methods. To solve this challenge, each process must be examined to ensure that viral clearance steps do not compromise the final product.
Inactivation typically utilizes extreme physical (pH, temperature) and/or chemical (detergents, solvents) conditions (30). Chemical processes are typical, as with poliomyelitis and influenza viruses, and often utilize β-propylactone (BPL) or formalin (formaldehyde). Insect cell-based processes can produce 1010–12 baculovirus particles that must be removed during downstream purification. As a safety measure, some manufacturers inactivate the baculovirus prior to removal through various combinations of BPL and high-temperature or solvent/detergent treatments. In killed-virus processes, the inactivation step aimed at the antigen can also inactivate other contaminant viruses.
In adeno-associated virus (AAV) processes, any replication-competent helper adenovirus must be inactivated and removed during downstream purification. AAV particles can resist heating at 52 ⁰C for 10 minutes, while human adenoviruses are more sensitive (31). Such treatment is effective but remaining denatured helper virus proteins may induce a cellular immune response in the patient and require removal by other methods.
Filtration is a robust method for virus removal in biologics production. However, filter size must be carefully selected in accordance with the size of the virus of interest and known and potential non-product viruses. Increasing evidence shows that removal of non-product viruses from viral-vectored vaccines or baculoviruses from VLPs expressed in insect cells can be achieved using 50-nm or 35-nm virus-retaining filters (32). This filtration, however, might not be applicable if the viruses share a similar diameter. Moreover, conditions should be adjusted to avoid aggregation or complex formation of therapeutic viruses and, thus, product loss.
Chromatographic methods are potentially effective tools for adventitious agent clearance from vaccine viruses. Chromatography has been shown to remove PCV (33), and ion exchange and affinity chromatography have been shown to purify vectors such as AAVs. However, because achieving robust clearance is challenging using chromatography, regulatory documents urge caution but remain encouraging. European guidance states that if the virus reduction is reproducible and the influencing manufacturing parameters are well defined, chromatography could fit the criteria of an effective step (34). Chromatography processes can be optimized to separate the product from helper viruses (35). Chromatographic separation of empty versus full capsids with a similar charge remain a challenge. Density separation using ultracentrifugation is possible but is challenging at GMP manufacturing scale.
Conclusion
Viral contamination of vaccines, albeit rare, can lead to serious human health and economic consequences. Regulatory and industry expectations vary in their specifics but do entail viral safety risk assessment and mitigation in the production of these therapeutics. A three-pronged approach—verifying the safety of raw materials, monitoring process intermediates for unintended viruses, and removing viral threats from products—can help prevent the administration of adventitious virus-contaminated viral vaccines and vectors to patients.
References
1. P.P. Pastoret, Biologicals38 (3) 332–334 (2010).
2. R.L. Garnick, Dev Biol Stand88, 49–56 (1996).
3. Genzyme, “Genzyme Reports Progress Related to Allston Plant,” Press Release, June 25, 2009.
4. A. Oehmig et al., J Gen Virol, 84 (Pt 10) 2837–2845 (2003).
5. Y. Qiu et al., Biotechnol Bioeng, 110 (5) 1342–1353 (2013).
6. J. Victoria et al., J Virol84 (12) 6033–6040 (2010).
7. H. Ma et al., J Virol88 (12) 6576–6585 (2014).
8. D. Mackay and N. Kriz, Biologicals, 38 (3) 335-337 (2010).
9. FDA, Guidance for Industry Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications(Rockville, MD, 2010).
10. WHO, Recommendations for the Evaluation of Animal Cell Cultures as Substrates for the Manufacture of Biological Medicinal Products and for the Characterization of Cell Banks, Technical Report Series 978 Annex 3(Geneva, Switzerland, 2013).
11. EDQM, EurPh, General Text 9.3; 5.2., “Cell substrates for the production of vaccines for human use,” (EDQM, Strasbourg, France, 2018).
12. CFR, 9 Code of Federal Regulation, Part 113(Government Printing Office, Washington, DC), pp. 47, 53.
13. CBER, Guidance for Industry—Cell Substrates and Biomaterial(Rockville, MD, Feb 2010).
14. EMA, Guideline on Influenza Vaccines(London, July, 2016).
15. EMA, Guideline on Quality, Non-Clinical & Clinical Aspects of Live Recombinant Viral Vectored Vaccines(London, June , 2010).
16. EMA, Guideline on Quality, Non-Clinical and Clinical Aspects Microbial Contamination of Gene Therapy Medicinal Products(London, May 2015).
17. EMA, Guideline on the Use of Bovine Serum in the Manufacture of Human Biological Medicinal Products(London, May 2013).
18. EMA, Guideline on the Use of Porcine Trypsin(London, February 2014).
19. P.M. Tomasula et al., J Dairy Sci.90 (7) 3202–3211 (2007).
20. M. Murphy et al., Biologicals39(6) 438–43 (2011).
21. M. Schleh et al., Am. Pharm.13, 72–76 (2010).
22. K. Remington and C. Hernandez, GEN34 (5) (2014).
23. W.A. Hijnen et al., Water Res.40(1) 3–22 (2006).
24. Q.S. Meng and C.P. Gerba, Water Res30, 2665–2668 (1996).
25. G. Chevrefils et al., IUVA News8 (1) 38–45 (2006).
26. H. Stettmund Von Brodorotti and H. Mahnel, Zentralbl Bakteriol B 170 (1–2) 71–81 (1980).
27. C. Carbrello et al., “Cell culture Engineering XV,” presentation at Engineering Conferences International (Palm Springs CA, 2016).
28. J. Gombold, Vaccine32 (24) 2916–2926 (2014).
29. C. Braxton, American Pharmaceutical Review(2017).
30. B. Sanders et al., “Inactivated Viral Vaccine,” in Vaccine Analysis: Strategies, Principles and Control, B. Nunnally, V. Turula, and R. Sitrin, Eds. (Springer, Berlin/Heidelberg, Germany, 2015), pp. 45–80.
31. B.A. Thorne et al., Biologicals36 (1) 7–18 (2008).
32. C. Bogedain, G. Maass, and M. Hoerer, “Filtration Method For Separating Viruses,” US patent 6479273. (Nov. 2002).
33. B. Yang et al., Biotechnol Prog.29 (6) 1464–71 (2013).
34. EMEA, Note for Guidance on Virus Validation Studies: The Design, Contribution and Interpretation of Studies Validating the Inactivation and Removal of Viruses. CPMP/BWP/268/95, (London, revised 1996).
35. G.J. Ye et al., Hum Gene Ther Clin Dev25 (4) 212–217 (2014).
About the authors
Anissa Boumlic*, PHD, is associate director EMEA vaccine and viral therapies, anissa.boumlic@emdmillipore.com; Martin Wisher, PHD, is senior director, global head of regulatory affairs for BioReliance; Damon Asher, PHD, is associate director Americas vaccine and viral therapies; and Priyabrata Pattnaik, PHD, is head of biologics operations—Asia Pacific; all are at MilliporeSigma.
*To whom all correspondence should be addressed.