A structured assessment process can determine compliance to lifecycle process-validation requirements for biopharmaceuticals.
The pharmaceutical regulatory landscape and pharmaceutical development have been reshaped by International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Harmonised Guidelines. ICH guidelines Q8–Q12 (1–5) and those in development such as ICH Q14 (6) have applied science, risk management, and quality systems to enhance process and product quality.
In 2011, FDA’s revised process validation (PV) guidance (7) extended ICH’s concepts to the pharmaceutical product lifecycle, an approach that is now being applied to cleaning validation and other areas of manufacturing, as well as to clinical trials (8).
This article proposes an independent structured assessment process that uses data to develop insights into product sustainability and risks. It focuses on the evaluation of intra- and inter-batch variability, and can be applied to both large- and small-molecule pharmaceuticals (9). This review process involves comprehensive process assessment (i.e., historical data review and the gathering of end-to-end manufacturing process data); extensive process monitoring and characterization exercises that enable lifecycle quality gaps to be addressed. The assessment process results in a heightened level of product and process knowledge, while substantially reducing the risk of potential failure.
Documents and methods
Based on the 2011 FDA process validation guidance and relevant ICH guidances, this assessment method requires the use of analytical and interpretive methods based on standard statistical and data visualization tools. The approach allows results to be verified at any time, by both product owner and regulator, utilizing standard statistical tools. All data sets prepared, transposed, and analyzed should be subject to data verification procedures to assure data accuracy and integrity.
Documents required for the assessment include the following:
- standard operating procedures
- master records
- executed batch records
- current specifications
- analytical test methods and test method validation
- analytical results
- annual product quality review outcomes
- investigation reports
- product recalls
- field alert report
- product complaints
- change control
- stability reports
- product development report
- submission dossiers
- process performance qualification (PPQ) protocols and reports
- equipment qualification reports.
The assessment uses current regulatory standards for validation (e.g., from FDA, the European Medicines Agency, Health Canada, and the World Health Organization). It also uses tools and methods such as cause-and-effect Ishikawa diagram, scientific rationale checklists, decision trees, as well as supporting analytical and statistical tools such as Minitab or JMP software.
The assessment focuses on analyzing the information and knowledge gained from the three stages of the process validation lifecycle: process design, process performance qualification, and continued process verification during commercial manufacturing. It is performed in the following two parts:
- Part A involves a holistic regulatory compliance review solely focusing on the compliance with the lifecycle-associated lifecycle regulatory guidance including ICH Q8, Q9, and Q10, and future industry trends including ICH Q12, Q13 (10), Q14 and how they work in tandem.
- Part B is a diagnostic study of the current process control (a seven-step product assessment process) that results in determining the adequacy of the current control strategy.
Part A. Compliance review: Adherence to FDA process validation and ICH guidelines
A comprehensive compliance review is designed to summarize and confirm compliance with the FDA process validation guidance and ICH and other emerging guidance, based on observation and the available data. The recent ICH Q12 document allows use of lifecycle guidelines for seamless post-approval change management. Adherence to lifecycle guidance therefore becomes more important for ongoing operational flexibility and compliance.
Stage 1, process design assessment, involves the following steps:
- determining the quality target product profile and justification of critical quality attributes (CQAs) and critical process parameters (CPPs) as per ICH Q8
- applying quality risk management tools as per ICH Q9
- documenting a critical manufacturing equipment suitability assessment
- determining degree and understanding variability
- documenting the product control strategy
- determining effects of scale.
Stage 2, process performance qualification review covers aspects such as the following:
- design considerations of a facility and qualification of utilities and equipment
- validation of analytical methods per analytical quality by design (QbD) as per ICH Q 14
- utilization of cumulative data from all relevant studies to establish the PPQ manufacturing conditions
- number of batches and samples to provide sufficient statistical confidence
- application of statistical process performance indicators
- clear documented justification for manufacturing process to be in a state of control.
Stage 3, continued process verification of the product includes the following:
- Continued monitoring and sampling of CPPs and quality attributes is at the level established during the process qualification stage until sufficient data are available.
- An ongoing program is in place to collect and analyze product and process data, detecting unplanned departures from the process.
- The data collection plan and statistical methods to determine process stability and capability are adequate in identifying variability in the process to signal potential process improvements.
- Review of failures, non-conformances, and trends during commercial manufacturing are conducted.
Part B. Control strategy review: The seven-step legacy product assessment
The seven steps involved in Part B are as follows:
Identify sources of variability (Step 1)
Variability is caused by contributing factors associated with materials, methods, machine, measurement, manpower, and mother nature (11). In manufacturing, this relates to attributes attributes of material, formulation, and associated processing parameters including scale-up factors, processing equipment, test method, personnel, and facility/utilities (Figure 1). The overall CQA variability is a function of individual component variability.
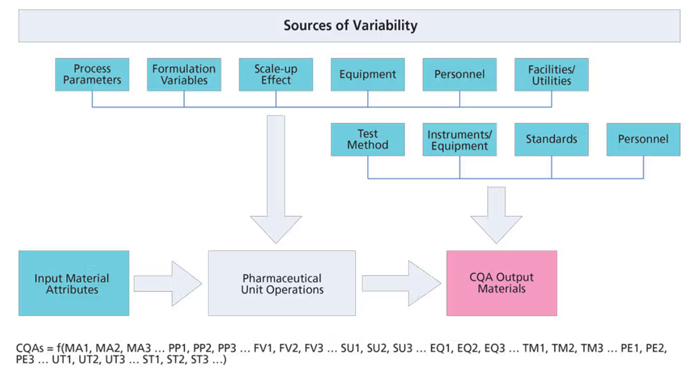
Figure 1. Sources of variability. CQA is critical quality attribute. Figures courtesy of the authors
A cause-and-effect technique is utilized to identify underlying factors that could have an impact on CQA that would affect patients (i.e., label claim CQAs). The assessment includes factors and attributes of equipment, personnel, and facility/utilities as show in Figure 2.
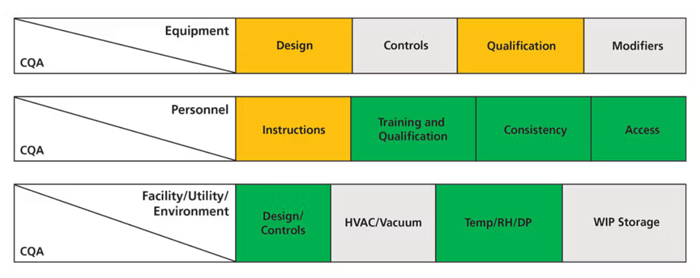
Figure 2. Example of risk assignment for equipment, personnel, and facility/utility/environmental factors. CQA is critical quality attribute. WIP is work in progress.
Criticality assignment (Step 2)
Upon identifying the risk factors through cause-and-effect techniques, a risk-rating decision tree (Figure 3) is used to analyze the process upon risk assessment. The criticality assignment will be commensurate with the product/formulation characteristics. In addition to any product-specific CQAs that would affect the patient, the review should—at a minimum—assess the impact of variability factors on CQAs. Risk assessment must cover all attributes.
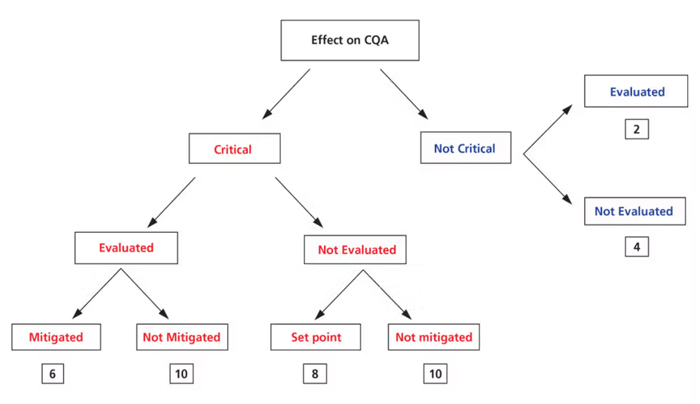
Figure 3. Risk rating method.
The decision tree with a data-driven semi-quantitatively risk-rating system (Figure 3) is used to prioritize the unexamined influencing factors in yellow (Figure 2) identified for each element. Additional review is warranted when the rating value is higher.
Gap assessment (Step 3)
The decision tree-based assessment (Step 2) determines whether the established current control strategy for each attribute is supported by the cumulative documented data from relevant Stage 1 studies (e.g., design of experiments, development batches). The control strategies established to control and mitigate the identified risk factors are then reviewed to determine the missing parts.
This gap assessment also evaluates whether the established current control strategies are derived based on objective information/rationale and data from original and subsequent process design studies. The need for establishing additional controls, including manufacturing process controls, is determined at the end of the gap assessment.
The gap assessment also determines whether the established current control strategy is supported by the cumulative data from all relevant studies (e.g., designed experiments, development batches, and PPQ batches). The control strategies established to control and mitigate the identified risk factors are reviewed to arrive at the conclusions. The residual risks and the need for establishing additional controls, including manufacturing process controls, are then identified to initiate a heightened sampling and testing Stage 3a plan for the product.
Lifecycle review and Stage 3a monitoring batch execution (Step 4)
The lifecycle review should determine, based on available lifecycle data, whether the current process is operating consistently within the originally established control strategy. The control strategy gap assessment step also may identify the need for additional controls or identify gaps in supporting data. The lifecycle review will determine if any commercial product data supports the identified gaps.
Data from all three stages of the process validation lifecycle (e.g., design of experiments, manufacturing trials, statistical analysis, annual product review, and commercial batch data) may enable the development of a risk-based control strategy that would suitably minimize sources of variability. Data from previous credible experience with sufficiently similar products and processes may also be utilized to support the control strategy.
Data gaps identified at Stage 1 QbD, Stage 2 equipment/facility/utility qualification and PPQ, and Stage 3 continued process verification require that a sampling and testing plan be developed under a Stage 3a protocol to further enable prediction with adequate statistically significant data sets.
The type of Stage 3a sampling/testing plan developed is based on the product/process gaps that have been identified. The Stage 3a study focuses on processes that have not been characterized, or for which variability has not been determined. Extensive data must be collected and tested and a monitoring protocol executed by the technical operations team responsible for Stage 1 lifecycle of the product. The Stage 3a lifecycle monitoring data that are collected supports the identified gaps or enables closure of existing Stage 1 data gaps for the product.
Data-driven, scientifically sound Stage 3a monitoring enables the following:
- continual enhancement of the product control strategy
- collection and evaluation of information and data, such that sources of variability can be detected, as recommended by FDA process validation guidance
- closure of the characterization gaps that were not addressed during early product development and to meet QbD standards, per ICH Q8 (R2)
- determination of intra- and inter-batch variability as recommended by FDA process validation guidance
- development of a baseline for Stage 3b monitoring as recommended by FDA’s process validation guidance.
ICH Q10 recommends management of product and process knowledge from development through commercialization and until discontinuation. The monitoring batches add to the body of knowledge on the product. FDA’s process validation guidelines allow for use of credible experience with sufficiently similar products; hence a bracketing approach for monitoring may be applied and conclusions can be used for control strategy enhancement of similar products.
The Stage 3a data collection protocol (12) developed after gap assessment enables in-depth statistical assessment of the product. The Stage 3a assessment will also provide a baseline for continual Stage 3b program for the product. The approach supports the organization’s ability to make science- and risk-based continual process improvement decisions for the product as well as to identify areas where QbD development should focus for similar manufacturing processes/products. The Stage 3a monitoring measures are developed in line with upcoming revisions to ICH Q2/Q14 and the proposed United States Pharmacopeia General Chapter <1220> framework for analytical QbD which requires identification of adverse trends, allowing proactive measures and facilitation of continued improvements and change control through continued monitoring.
Historical data collection Step (5)
The extensive data collection step includes batch data from development, process performance qualification, and commercial manufacturing. The new database will include potential variables that can impact the CQAs. This will include, but will not be limited to, materials, upstream and downstream process parameters, equipment parameters, hold times, operational variables, operation/analytical personnel, instrumentation, documented weights/time, facility/utility parameters, and incidents. This step will involve extensive data set collection, data set review, and data preparation for the subsequent statistical analysis.
As a result, the database must be developed so that it maximizes the utilization of existing product/process data for multivariate statistical analysis such as correlations. The database is created for variables that can impact CQAs. The database is utilized as a starting point for Stage 3b trending and ongoing signal detection of products that are determined to be robust post assessment.
Statistical analysis (Step 6)
The data collected from Stage 3a heightened sampling and the historical batches are statistically analyzed to identify patterns and insights into the impact of potential sources of variability on the CQAs. Statistical software tools such as Minitab and JMP may be utilized. The analysis determines the adequacy and suitability of the collected data sets to support the current control strategy. The analysis first attempts to determine whether the original control strategy developed adequately meets the current standards.
Enhancement requirements are then determined, based on the correlations and trends observed from batch data across commercial manufacturing. This step includes an assessment of within- batch and between-batch variability, and the associated controls available to minimize the variability. The statistical assessment can identify patterns and insights on impact of potential sources of variability
on the CQAs.
The statistical assessment tries to address each of the attribute gaps identified though Stage 3a heightened monitoring and analysis. The rating table is then updated based on the newly identified and generated data, as shown in Table I.
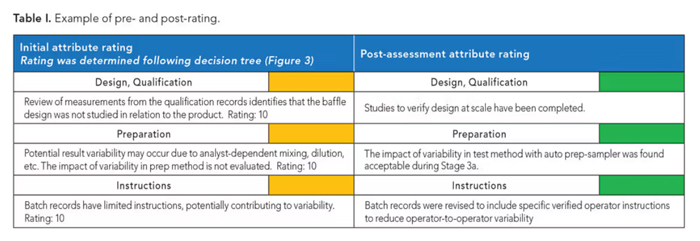
Table I. Example of pre- and post-rating.
Interpretation of results (Step 7)
Interpretation generates actionable recommendations based on the statistical analysis. The data-driven estimations allow for in-depth product/process understanding, proving product robustness, and are in line with regulators’ expectations that science and data be used for product and process decision making. The determination is based on data signals identified through assessment of the historical data and the generated heightened results from Stage 3a monitoring batches.
When adequate data are available, the signals seen (i.e., CQAs, key performance parameters and CPPs) can be categorized based on risk. All signals are not created equal, and different action plans are required for the yellow flags. The assessment suggests actions based on practical relevance and the statistical strength of the signal (13). As a result, this review process enables organizations to make data-driven justification on the product sustainability.
Conclusion
The protocol-driven product assessment closely dissects the manufacturing process to determine the current state of control, to comprehend and address gaps for completeness of validation status. The exercise also determines the process robustness, allowing for business decision making. The review provides clear data-driven input for one of three actions:
- Developing a Stage 3b monitoring program, in cases where product has been determined to be robust,
- Developing a Stage 1 continuous improvement plan, or
- Developing a Stage 1 remediation plan.
The seven-step plan serves as the first concrete step in commercialized product risk reduction. The combination of the substantial lifecycle data and the newly generated Stage 3a data (where required) allows for multivariate analysis, and establishes an updated design space for the product. The seven-step robustness assessment can be considered an exercise contributing towards business continuity and a source of great value for organizations.
References
1. ICH, Q8 (R2) Pharmaceutical Development, Step 4 version (2009).
2. ICH, Q9 Quality Risk Management, Step 4 version (2005).
3. ICH, Q10 Pharmaceutical Quality Systems, Step 4 version (2008).
4. ICH, Q11 Development and Manufacture of Drug Substance, Step 4 version (2012)
5. ICH, Q12 Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management, Step 4 version (2019).
6. ICH, Q14 Analytical Procedure Development and Revision of ICH Q2 (R1) Analytical Validation, Final Concept Paper (2014).
7. FDA, Guidance for Industry, Process Validation: General Principles and Practices (CDER, January 2011).
8. ICH E8 (R1) General Considerations for Clinical Studies, Step 2b version (May 2019).
9. A. Pazhayattil, et. al., Pharm Tech, 45 (7) 54–62 (July 2021).
10. ICH, Q13 Continuous Manufacturing of Drug Substances and Drug Products, Step 1 version, (June 2018).
11. L. Liliana, “A New Model of Ishikawa Diagram for Quality Assessment,” presentation at the 20th Innovative Manufacturing Engineering and Energy Conference Series (2016).
12. S. Sharma, et al., “Lifecycle of Legacy Powder fFor Oral Suspension: Stage 3 Validation Monitoring”, Journal of Validation Technology (2021).
13. A. Pazhayattil, et. al., “Continued Process Verification: Reacting to Data Signals”, PDA Letter (Nov. 18, 2020).
About the authors
Ajay Babu Pazhayattil is lead consultant, specializing in process validation, with Validant. Sanjay Sharma is vice-president and head of technology transfer at Lupin Pharmaceuticals. Amol Galande is senior manager of the process development laboratory at Lupin Pharmaceuticals. Marzena Ingram is senior consultant at Validant. Robert Rhoades is managing partner at Validant.