The necessity to detach cells from a culture substrate during cell harvesting remains one of the most challenging steps in a cell-culture process.
Cell culture is widely employed in biomedical applications and has numerous applications, spanning from diagnosis, therapy, and the production of biological drugs. Cells used for biopharmaceutical applications are mainly of animal origin and can be cultured in suspension or adherent cell cultures. A unique characteristic of adherent cells is their dependence on an anchorage surface on which to attach to exert their normal metabolic activity and proliferate. While cell attachment may prove advantageous for some purposes, such as supernatant harvesting, cell detachment may become an enormous challenge when cells are to be harvested. Cell detachment is probably one of the most relevant processes hindering a faster development for cell-based biotechnology. In this article, the authors focus on different strategies available for animal adherent cell detachment.
Animal adherent cell cultures are derived either from tissue explants or from cell suspensions. In a standard culture process, once cells have attached to the culture support, they undergo a lag phase and then start growing exponentially at a high metabolic activity, until confluency is reached. Then, growth stops and the culture reaches a stationary phase. Most often, cells are harvested when population density suppresses growth. The on-demand release of cultured cells, however, would be greatly beneficial for certain biomedical applications in which a certain development step of the living cells is pursued, rather than simply the pursuit of a large number of collected cells. This release should be efficient and triggered by a simple stimulus.
Choice of cell-detachment method: Factors to consider
Cell behavior and the degree of adhesion of cells to the culture support are greatly affected by several culture parameters. However, adhesion degree is not the only factor to be considered when choosing the most convenient cell detaching method within a given cell-culture process. The authors consider different factors and requirements that influence the choice of the right cell-detachment method.
Degree of cell adhesion
Vigorous cell-culture processes, such as culture on microcarriers, require strong cell adhesion that needs be matched by the harshness of the detaching step. On the other hand, cell culture on t-flasks is more suitable for cells that establish weak interactions with the support.
Further use of detached cells
When subsequent reattachment is expected from detached cells (e.g., in a subpassage step during cell expansion or for therapeutic cells production), cell viability and membrane component integrity are more important than in the case of cell harvesting for further preparation of cell extracts. Similarly, when a cell sheet is to be obtained, cell-to-cell interactions must remain intact, but not when cell suspension is the target product. In addition to physical integrity of the cells, an optimal functionality/metabolic state of such cells is also desirable.
Process compatibility
Some chemicals necessary for particular cell-detachment procedures may interfere with subsequent downstream processing steps that are unaffected by other alternative detachment methods. Therefore, detachment based on physical processes is certainly preferable to chemical treatments, and help mitigate concerns related to the impact of process additives on a final product.
Culture support
Cell-detaching methods where direct access to the support is necessary, such as cell scraping, are not compatible with cell-culture supports such as microcarriers or hollow fibers.
Process scale
Manual cell-detachment techniques such as cell scraping may be adequate at laboratory scale but unfeasible at industrial scale due to the laboriousness of the method.
Reusability of culture substrates
Some responsive substrates designed to promote cell detachment under the effect of a particular stimulus undergo an irreversible structural modification that prevents repeated cell culture on the same substrate.
Regulatory constraints
Regulatory restrictions may exclude some cell-detachment methods from consideration in particular applications or require extensive validation before authorization of use is granted.
Spatial resolution
Applications such as co-culture of different cells in a given geometrical pattern or single-cell harvesting require the ability to detach cells only from selected areas without affecting cells growing in other areas of the support.
Temporal resolution
Some stimuli used to promote cell detachment are deleterious for cells. In these cases, it is important to control the temporal resolution of the cell-detachment technique.
Compatibility with sterilization methods
In case sterilization of the cell culture device is necessary prior to use, care should be taken to ensure that the sterilization method does not affect the characteristics of the cell detachment technique (e.g., when using supports with modified surfaces).
Shelf-life
Cell-culture devices are often provided as ready-to-use devices that are stored for a long period of time before use. Some components of chemical and biological origin contained in sophisticated cell-detachment systems are unstable and have a short shelf-life.
Production costs
Expensive cell-detachment systems negatively affect the overall cell-culture process cost and determine the applicability of the technique.
Alternative cell-detachment methods
For many years, treatment with the protease enzyme trypsin has been the standard method for cell detachment. This method, widely named trypsinization, is based on the addition of an active concentration of the enzyme to the cell culture and subsequent digestion of cell-membrane proteins that establish the interaction with the support surface. Trypsinization variants have been developed along the years to try and circumvent the drawbacks associated to the deleterious effect of this enzyme on the cells and other limitations derived from the chemical nature of the stimulus employed to disrupt the interaction of the cells with the support.
The successful development of biopharmaceuticals produced from animal cell culture and other applications of adherent cells in the biopharmaceutical industry has spurred research on alternative cell-detachment methods that address deficiencies of the trypsinization technique and help realize the huge potential of adherent cells to enable therapeutic and diagnostic solutions.
Cell attachment requires the interaction of cell membranes with the culture support, and in order to distort this interaction, different cell-detachment methods use different types of stimuli to target either the cells, the membrane support, or both. Besides, the effect of different stimuli on the target may have a different degree of reversibility, having an impact on the applicability of the different cell-detachment alternatives.
The following is a brief description of these alternative cell detachment methods, currently on different degrees of development, with potential impact in biopharmaceutical applications. This information is summarized in Table I.
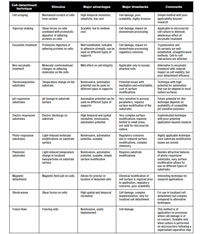
Table I: Cell-detachment methods with potential impact on biopharmaceutical applications.
In cell scraping, a rubber or plastic spatula is used to physically remove the cells from the culture support. This manual method based on a mechanical stimulus is limited to devices with a smooth culture surface accessible to the laboratory technician and has important automation limitations. The method is quick and easy when performed in a reduced number of devices but disruptive to the cells and may result in significant cell death. The absence of chemical reagents, surface modifications, or complex equipment, however, makes this technique an attractive alternative for laboratory scale cell detachment from t-flask-like culture devices.
An alternative to cell scraping for use in microcarrier-based cell culture is the combination of vigorous shaking with a mild trypsinization treatment (1). This cell-detachment technique combines two types of stimuli: mechanical and chemical. The method also has the potential for scalability. Careful analysis of the combined effect of both stimuli on the cells and on the subsequent processing of the product, however, is necessary. Apart from concerns raised by the use of proteolytic enzymes, this method is easy to implement in large-scale microcarrier cell culture. A variation of this method exploits the strong shear forces in microfluidic systems (2). In such a method, cells are cultured on the internal walls of channels in microfluidic chips. When a strong liquid flow is passed through the microfluidic channels, the cells are subjected to a high shear force that provokes an efficient detachment. This method is considered a harsh detachment procedure, however, often resulting in significant damage and even death of the cells.
Enzymatic treatment with alternative proteolytic enzymes such as collagenase or Pronase, a commercially available mixture of several nonspecific endo- and exoproteases produced by Streptomyces griseus, are alternatives to the traditional trypsinization method. These enzymes digest proteins exposed in the cell surface to distort the interaction of the cells with the culture support. Enzymatic activity on the cell surface can be deleterious to the cells and irreversible damage such as the apoptotic effect induced by the trypsin treatment (3) can be expected from other proteases. This method has poor temporal resolution, leading to extended activity of the enzyme on the cells and subsequent cell damage. Strong regulatory considerations are also associated with the use of enzymes in biopharmaceutical applications. On the other hand, this method provides an efficient way to disrupt cell-to-cell interactions, a useful feature of interest for harvesting cell suspension rather than cell sheets.
Non-enzymatic chemical treatment is also an option. Enzymatic treatment is usually combined with Ethylenediaminetetraacetic acid (EDTA), a chelating agent for divalent cations that helps trypsin do its task and inhibits the interaction of some of the proteins involved in cell-to-cell interactions and cell-to-support interaction. EDTA is also applied alone when cells are loosely attached to the culture support. The citric saline method (4) has also been reported as a very gentle treatment for cell detachment. Non-enzymatic chemical treatment provides a gentle cell-detachment method. Besides poor spatial and temporal resolution, these methods suffer from poor performance in terms of the ratio between required amount of chemical and cell detachment. Therefore, applicability of this technique is restricted to a limited number of cell lines.
Thermoresponsive substrates have been used to promote the detachment of adhered cells. In this method, the cell-culture support is coated with a thermoresponsive polymer such as poly(N-isopropylacrylamide) (pNIPAAm) following different chemical strategies. Cells are cultured on the pNIPAAm-coated support, and cell detachment is achieved by dropping the temperature of the culture below the lower critical solution temperature (LCST) of pNIPAAm (32 °C). Below LCST, pNIPAAm undergoes a phase transition from a shrunken state to a swollen state that induces cell detachment due to changes in the hydrophobicity of the polymer. This strategy, initially applied to the recovery of cell sheets from t-flask type culture devices (5), has been extended successfully to microcarrier-based cell cultures (3). A key issue in this approach is raised by regulatory concerns associated with the use of current thermo-responsive polymers (6). Chemical synthesis of such polymer brushes onto the surfaces of cell-culture supports requires a number of steps and reagents that might hinder their applicability. Otherwise, these systems are interesting due to their potential for automation, scalability, and noninvasive cell detachment.
The use of pH-responsive polymers grafted on cell-culture supports has allowed researchers to promote cell detachment by lowering environmental pH from 7.4 to 6.5 (7). Cell detachment can occur following structural changes that take place in the pH responsive polymers upon pH decrease. Changes in the polymer charge from positive to negative in response to a pH increase have been used to detach cells from chitosan-coated supports (8). This method could potentially be employed to detach cells from microcarriers or from t-flask-like cell culture devices, but similar to thermoresponsive substrates, treatment with chemicals, and enzymatic treatment, requires the homogeneous modification of the overall culture environment conditions, which could result in poor temporal resolution.
Sophisticated gold-coated electro-responsive substrates have been employed to hydrolyze—under a transient electrical potential—the ether bond that retained the cell-adhesive ligands to which cells are attached (9). In this example, the complex structure of the system is a drawback for large-scale and mass production. Besides, the reaction is irreversible, because the cell-adhesive ligand molecules remain bound to the cells after the ether bond is hydrolyzed. Moreover, the applied electric potential might induce parallel electrochemical reactions. Despite the aforementioned drawbacks, the potential automation of the system may result in future efforts to further develop electro-responsive systems.
Photo-responsive substrates based on different designs and with different features have been studied. Some of these systems are based on changes on the hydrophilicity of the substrate by light illumination (10). Some are based on reversible structural changes in molecules grafted on the surface of the support (11), and others use the irreversible cleavage of a photolabile linker to release a cell-adhesive molecule bound to the substrate (12). In all cases, the high spatial and temporal resolution of light sources is an important advantage of these cell-detachment systems. Some of these systems induce an irreversible modification on the substrate or a surface modification on the cell membrane. Complex modifications of the substrate are necessary to sensitize culture supports and prepare them to respond to light. Otherwise, use of light as stimulus for cell detachment is one of the most promising alternatives for automated, noninvasive cell detachment for biopharmaceutical applications, including large-scale production of biopharmaceuticals using different types of culture supports.
Plasmonic substrates incorporate a particular type of light-induced cell-detachment properties. In this type of substrate, a transient hyperthermic effect results from the interaction of light of a given wavelength with nanoparticles integrated in the support. To date, available systems were based on the use of near-ultraviolet light, resulting in an aggressive treatment for the growing cells and in loss of spatial and temporal resolution due to the formation of reactive oxygen species (ROS) that diffuse in the culture medium (13). Efforts in this direction have led to the development of a plasmonic substrate that promotes cell detachment upon a short illumination with near-infrared (NIR) light (14). This technique is based on plasmonic substrates produced by embedding gold nanoparticles on the surface of the cell-culture support. The controlled size and geometry of the nanoparticles, as well as their close contact, leads to an intense plasmonic phenomena upon interaction with NIR light—better suited for cell treatment than ultraviolet light—widely known to induce mutagenic alterations in cellular DNA. Besides, the system combines the attractive properties of light as a controlled stimulus with a simple and robust support readily transferable to different cell culture devices. Most advantageously, NIR light can be applied from outside the cell-culture device, thus providing a noninvasive cell-detachment system. The effect of NIR light on cells growing on plasmonic substrates is shown in Figure 1.
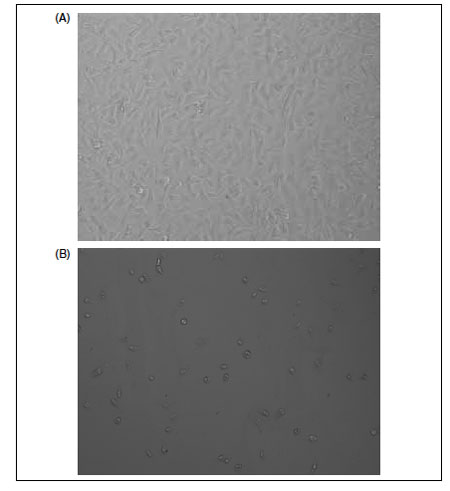
Figure 1: Cell detachment from plasmonic substrates. Transmitted light images of HeLa cells grown on plasmonic substrates before (A) and after (B) irradiation with a near-infrared laser at 980 nm. Power density of 340 mW/cm2 during 20 min.
Cells labeled with magnetic nanoparticles or liposomes have been used to demonstrate the viability of magnetic systems for cell detachment. In these systems, cells lightly adhered on the culture support are treated with positively charged liposomes that readily bind to the negatively charged cell membranes (15). Then, a magnetic field is applied to detach the cells from the culture surface. The good spatial and temporal resolution associated to the localized application of the magnetic field and the possibility to deliver detached cells to designated locations are of potential interest. However, the complexity associated to this method and other considerations, such as the specific features required for the cell membrane to interact with the liposomes, preclude its application beyond dedicated research experiments.
Shock waves originated from a piezoceramic source adapted from a commercial lithotripter have been used to detach adherent cells (16). In this work, the ability of surviving detached cells to reattach and propagate was not assessed. However, cells detached by laser-induced shockwaves have been shown to adhere again (17). This technique has good spatial and temporal resolution. There are few advantages to this complex and shear-intensive technique, however, when applied to the culture of adherent cells for biopharmaceutical applications.
For particular applications that are more related to research and production of cell fragments, freeze/thaw is a traditional method for cell detachment, where cell viability is not of major concern (18). This method has been proposed for cell harvesting from microcarriers (19), especially for harvesting protease-sensitive biological materials, and it is often used to obtain cell fragments. Freeze/thaw has a detrimental effect on cell viability and integrity and a poor temporal resolution. On the other hand, regulatory concerns related to this technique are scarce.
Conclusion
Some of the discussed methods for cell detachment are already available commercially, while others are in different stages of development. In coming years, the authors expect to see commercial variants of these methods implemented in existing devices for cell culture, as is the case of the ongoing efforts of the authors to incorporate plasmonic cell-detachment features into the Bolt-on bioreactor (20). Refinement of the methods that have great potential for enabling efficient cell detachment is also necessary, as many of the newer techniques are still too complex and/or sophisticated to be widely used by the cautious biopharmaceutical industry.
References
1. W.N. Nienow et al., Biochem. Eng. J. 85, pp. 79–88 (2014).
2. K.W. Kwon et al., Lab Chip 7, pp. 1461–1468 (2007).
3. H.S. Yang et al., Cell Transplant. 19, pp. 1123–1132 (2010).
4. R. Pape et al., Arterioscler. Thromb. Vasc. Biol. 28, pp. 534–540 (2008). DOI: 10.1161/ATVBAHA.107.159483.
5. H.E. Canavan et al., J. Biomed. Mat. Res. Part A 75A (1), pp. 1–13, (2005). DOI: 10.1002/jbm.a.30297
6. M. Patenaude and T. Hoare, “(302c) Injectable, Degradable Thermoresponsive ploy(N-isopropylacrylamide) Hydrogels,” presentation at the 12th Annual Meeting of the AIChE (2012).
7. X.-Q. Dou et al., Soft Matter 8, pp. 9539 (2012).
8. Y. Chen et al., Biomaterials 33 (5), pp. 1336–1342 (February 2012). doi:10.1016/j.biomaterials.2011.10.048
9. W.-S. Yeo et al., Chembiochem 2 (7–8), pp. 590–593 (Aug. 3, 2001).
10. Y. Hong et al., Biomaterials 34 (1), pp. 8–11 (January 2013). doi:10.1016/j.biomaterials.2012.09.043
11. A. Higuchi et al., Biomacromolecules 5, pp. 1770–1774 (2014).
12. M. Wirkner at al., Adv. Mater. 23 (64), pp. 3907–3910 (2011) DOI: 10.1002/adma.201100925.
13. T.A. Kolesnikova et al., ACS Nano 6 (11), pp. 9585–9595 (2012).
14. J.J. Giner-Casares et al., Angew. Chem. Int. Ed. 55, pp. 974–978 (2016).
15. A. Ito et al., Tissue Eng. 10 (5-6), pp. 873–880 (May–Jun 2004).
16. C.-D. Ohl et al., Biochimica et Biophysica Acta 1624, pp. 131– 138 (2003).
17. Y. Hosokawa et al., Appl. Phys. A 79, pp. 795–798 (2004).
18. N. Nishishita et al., Am. J. Stem Cells 4 (1), pp. 38–49 (2015).
19. V.V. Ranade and J.B. Cannon, Drug Delivery Systems, Third Edition, (CRC Press, April 25, 2011).
About the authors
Marcos Simon, PhD is founder of The Bolt-on Bioreactor project and Technology Transfer Manager at CIC biomaGUNE.