By Cristina Baccarelli, Paola Bernard, Teresa Cortellino, Oscar Cruciani, Rita Pacello, Chiara Parisi, Luisa Stoppa, Isabella Marta
This article discusses cleaning validation of equipment dedicated to the production of a single API.
Cleaning of pharmaceutical equipment is essential to reduce the risk of product contamination and, as stated in relevant guidelines and as recognized by the pharmaceutical sector, this can be achieved only if the cleaning procedure has been validated. International Conference on Harmonization (ICH) guidance ICH Q9 (1) encourages that a quality risk management approach be considered and that, based on the level of risk, cleaning processes may be subject to different levels of validation or verification.
This article covers cleaning validation of equipment dedicated to the production of a single API; equipment used for manufacturing a class of products (e.g., penicillins) should be considered as shared equipment and is not addressed here.
Generally speaking, when one thinks of cleaning validation, the first thing that comes to mind is “prevention of cross-contamination”, which obviously applies only when equipment is used for manufacturing more than one product. So why is cleaning validation talked about with regard to dedicated equipment? Section 12.70 of the guideline ICH Q7, states that, “Cleaning procedures should normally be validated. In general, cleaning validation should be directed to situations or process steps where contamination or carryover of materials poses the greatest risk to API quality…” (2). Table I highlights the differences between the approach to clean shared and dedicated equipment.
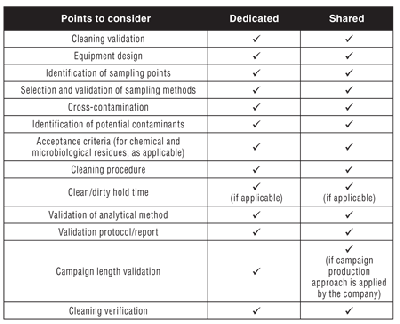
Table I: Differences between cleaning of dedicated and shared equipment.
As indicated in Table I, most points apply to both cases, meaning that great care needs to be given also when planning cleaning validation activities of dedicated equipment.
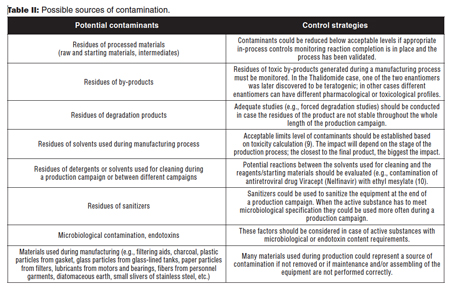
For dedicated plants/equipment, there is no risk of cross-contamination among different active substances; nevertheless, a wide range of possible contaminants must be evaluated on a case-by-case basis (3), taking into consideration the type of process (i.e., chemical synthesis, extraction from natural sources, fermentation, physical steps, etc.), the final product, and the materials used during the manufacturing process (i.e., starting and raw materials, solvents, and reagents). As for cleaning validation of shared manufacturing plants, even for dedicated plants/equipment, it is necessary to identify all possible sources of contamination. Some key points to be considered are summarized in Table II.
Only cleaning procedures that have been validated ensure that any undesirable residues have been effectively removed below a level that has been demonstrated to be acceptable and that does not pose a risk to patients. Cleaning validation is time and resources consuming; however, some companies might prefer not to use shared facilities and, instead, dedicate an entire building, manufacturing line, or piece of equipment for the manufacture of a single product. If they use disposable equipment, such as single-use bioreactors, compatibility of the disposable equipment with the process should also be assessed.
Companies manufacturing only one product use dedicated equipment by default. In addition, companies must use a dedicated facility, line, or equipment, if:
• The calculated limits of undesirable residues are too low and therefore the potential residues would not be detectable with the available analytical methods (2).
• The acceptance criteria are too low and cannot be achieved (i.e., for some high potency active substances).
• The data to calculate safe threshold levels for toxic or sensitizing substances are not yet available or not sufficient (4).
Planning for cleaning validation
Visual examination is not acceptable as the only test to check residue during initial validation studies. Even if some literature data report proposed visual limits, this test does not meet ICH Q7 expectations, as it may depend on too many variables that are difficult to standardize (5) and validate. After the cleaning validation has been performed, visual examination could be used to detect “gross contamination” of the equipment immediately before use (2).
As for non-dedicated facilities, equipment should be of appropriate design and adequate size and suitably located for its intended use, cleaning, and sanitation (2). Design and technical aspects of equipment are covered by good engineering practices (6) and some technical references; practical guidelines for equipment design are also provided by food industry regulation (7). Equipment can be considered as “cleanable” if it is constructed using adequate materials such as stainless steel or polymeric materials that are compatible with the process to be carried out. Finished surfaces should be smooth and properly polished, and equipment should be appropriately designed and assembled in a way that facilitates cleaning and prevents microbial growth (i.e., no dead legs, not too many horizontal pipelines or excessive instrumentation, and ancillary components such as shafts, bearings, and agitators should be easy to disassemble). Finally, the equipment should be easy to inspect.
Sampling method selection (swabbing and/or rinsing or other means [8]) is essentially related to type and design of the equipment, nature of the residues, residues acceptance limits, and the analytical methods used for residues quantification; the approach to sampling is the same for dedicated and shared facilities.
Cleaning validation of dedicated production equipment
There are three main aspects to consider when performing cleaning validation of dedicated production equipment: campaign length, clean hold time (CHT), and dirty hold time (DHT).
If cleaning of equipment dedicated to one API production is not carried out after each batch but on a campaign basis, it is necessary to validate the maximum campaign length (in terms of duration, number of batches, and batch size) by demonstrating that manufacturing consecutive batches with no cleaning between them does not lead to a build-up of undesirable residues that cannot be properly removed at the end of the campaign with the selected cleaning procedure.
Recent inspection results show that lack of cleaning validation related to the maximum campaign length is still an issue that is worth clarifying. During an inspection of a dedicated manufacturing facility, conducted by the Italian Medicines Agency, Agenzia Italiana del Farmaco AIFA in 2015, it was observed that no validation studies were performed to determine an appropriate campaign length (i.e., duration and/or number of batches that can be manufactured before having to clean the equipment). Moreover, the company failed to put in place intra-campaign controls aimed at verifying that during a production campaign the level of potential degradation residues in the equipment was maintained to a minimum and below pre-established specifications.
CHT is defined as the time between the completion of cleaning and the beginning of the next manufacturing operation. Clean equipment will not remain clean indefinitely depending on the length of the storage period and the condition of the storage environment. Cleaning validation studies, therefore, should demonstrate that storage conditions do not contribute to growth of microorganisms. Evaluations need to be performed on a case-by-case basis; a CHT might not need to be defined if, for example, the equipment is always cleaned just prior to use.
The CHT also must be determined for dedicated equipment/facility. During an inspection conducted by FDA, it was observed that tanks used for the manufacture of a single API, carried out a few months before, were not cleaned since the last campaign. The interior of the equipment had accumulated approximately half an inch of a white substance and contained a shallow pool of liquid at the bottom.
DHT is defined as the time between the end of manufacturing and the beginning of cleaning procedure. A residue easy to remove, if cleaned immediately after use of equipment, could maybe be difficult to remove when applying the same cleaning procedure if the cleaning was not performed immediately after use. This could occur, for example, for the residues in a crystallization tank; the “wet” residues might be easy to remove while the “dried” residues could require a different cleaning procedure. As for CHT, evaluations need to be performed on a case-by-case basis. A DHT might not need to be defined if, for example, the equipment is always cleaned right after use.
For the reasons mentioned previously, it can be concluded that it is crucial to conduct cleaning validation for dedicated equipment. The quality of an API is intrinsically related to the cleaning procedure employed; therefore, this aspect needs to be adequately addressed by the manufacturers and deeply reviewed by regulatory authorities during GMP inspections.
Reference
1. ICH, Q9 Quality Risk Management (ICH, Nov. 9, 2005).
2. ICH, Q7 Good Manufacturing Practice Guide For Active Pharmaceutical Ingredients (ICH, Nov. 10, 2000).
3. A. Walsh, Pharmaceutical Engineering (November/December 2011).
4. EMA/CHMP/ CVMP/ SWP/169430/2012, “Guideline on setting health based exposure limits for use in risk identification in the manufacture ofdifferent medicinal products in shared facilities”.
5. PDA, Technical Report No. 29 (Revised 2012), Points to Consider for Cleaning Validation (PDA, 2012).
6. ISPE, Good Practice Guide: Good Engineering Practice (ISPE, 2008)
7. BS EN 1672-2:2005 Food Processing machinery-Basic concepts.
8. EDQM, EudraLex, Volume 4, EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use, “Annex 15: Qualification and Validation” (in force from October 1, 2015).
9. ICH, Q3C (R5) Impurities: Guideline for Residual Solvents (ICH, Feb. 4, 2011).
10. M. C. Killilea, “Cleaning Validation: Viracept, 2007” IVT Network (Dec 3, 2012).
Editors’ Note: This article is based on a topic addressed during the 6th Pharmaceutical Inspection Co-operation Scheme (PIC/S) Expert Circle on APIs meeting, held in Rome in May 2014 and hosted by the Italian Medicines Agency (AIFA). PIC/S is a co-operative arrangement between 46 regulatory authorities worldwide active in the field of GMP for medicinal products for human or veterinary use. It aims at harmonizing inspection procedures globally by developing common GMP standards, training inspectors, and facilitating exchange of information and mutual confidence between regulators.





