In clinical monoclonal antibody (mAb) manufacturing, capture on protein A chromatography resin gives excellent recovery and purity. However, because clinical batch sizes are small, fewer cycles are required compared with full-scale manufacturing; this means that the full resin lifetime is rarely realized.
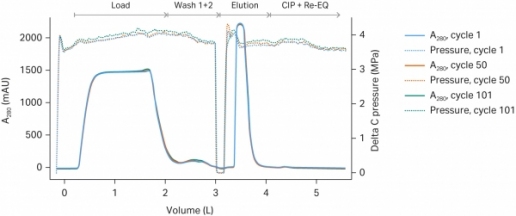
Bayer worked with Cytiva to evaluate a fiber-based alternative, Fibro PrismA, to determine its potential as a truly single-use mAb capture technology. The team assessed scalability from lab- to process-scale units, tracking pressure drop, step recovery, purity, and eluate volumes across multiple cycles.





