By: Anurag S. Rathore, Ranga Godavarti, Vijesh Kumar, Nihal Tugcu for BioPharm International
The authors discuss the evolution of the purification platform for manufacturing of mAb therapeutics.
Monoclonal antibodies (mAb) increasingly form the majority share of the product pipeline, as well as revenue, of major biopharmaceutical companies (1). What is unique about mAbs from a processing perspective is the applicability of developing a common process and analytical platform (2, 3). Some process development related attributes with respect to production of mAbs include:
- Significant increase in titer levels over the past decade (from 0.5 mg/mL to 5-10 mg/mL)
- High product requirement (as much as tons) due to higher doses of typical mAb products
- Larger manufacturing facilities (from 1-5KL to 10-20KL)
The efficiency of the platform process, as it enables process development (e.g., time taken, resources required), analytical development, and manufacturing, is quite significant, such as:
- Process development timelines can be significantly reduced
- Analytical development timelines can also be significantly reduced (use of standardized assays and platforms)
- Ease of scale-up and technology transfer due to similarity in the processes
- Reduced capital expense when bringing in a new product to the manufacturing facility.
It has been more than a decade since the industry started establishing the process platform. This 31st article in the “Elements of Biopharmaceutical Production” series focuses on evolution of the purification platform for manufacturing of mAb therapeutics.
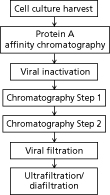
Figure 1: Illustration of the commonly used mAb downstream processing platform. Chromatography steps could be cation exchange chromatography, anion exchange chromatography, or hydrophobic interaction chromatography.
Traditional Monoclonal Antibody Platform
Figure 1 illustrates the traditional mAb platform that is commonly used (2,3). Typical steps include:
- Protein A chromatography for capture of the product and removal of host cell-related impurities (host cell proteins and DNA). Though extremely efficient and effective, the step primarily suffers from the significantly high cost of Protein A resins in comparison to other popular modes of chromatography (such as ion exchange). The low pH elution is another potential issue as it has been linked to product aggregation.
- Low pH viral inactivation as an orthogonal step for clearance of retroviruses. This step also suffers from the possibility of product aggregation at low pH.
- Cation exchange (CEX) chromatography is also quite commonly used. The primary objective of this step is to remove host-cell proteins, DNA, charged variants, and aggregates. Bind and elute mode is used to facilitate removal of product-related impurities.
- A second chromatography step is often used (either prior to the CEX step or following it) with the purpose of further removal of host-cell related impurities (e.g., host-cell proteins and DNA) or product-related impurities. Anion exchange (AEX) chromatography in a flow-through mode is the method of choice for removal of host-cell impurities. Hydrophobic-interaction chromatography (HIC) in bind and elute mode has been used to achieve further clearance of product-related impurities.
- Virus filtration step is the method of choice as another orthogonal step for virus removal.
Major Adaptations to the mAb Platform
Use of multiple washes in Protein A
To understand the resolution of fragments and aggregates on Protein A, a pH gradient elution between pH 5.0 and 3.0 over 10 column volumes was carried out, and fractions were collected for analysis via high-performance size exclusion chromatography (HPSEC). Based on the data presented in Figure 2, resolution between low molecular weight fragments and monomeric antibody could be achieved. These results led to inclusion of a two-step elution strategy where a high pH (4-5) wash followed by low pH (3-4) elution was used to minimize the fragment content in the Protein A product pool. When implemented for a specific product, the revised elution scheme led to a 5% absolute reduction in fragment content with a 10% yield loss.
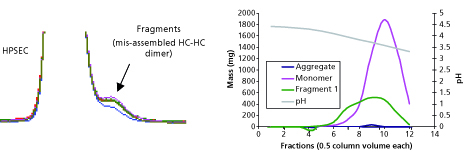
Figure 2: (a) HP-SEC profile showing the presence of a shoulder consisting of fragments, (b) chromatogram demonstrating resolution of fragments during Protein A with pH gradient. HP-SEC is high-performance size exclusion chromatography.
Gradient elution for cation exchange chromatography
Another modification to the traditional antibody purification platform is the inclusion of gradient elution during cation exchange chromatography (CEX). Typically, the elution for CEX is isocratic (constant salt and pH conditions). Under certain cases, however, this may lead to issues of robustness and other complications (4). During development of therapeutic candidates expressed in dhfr- CHO, for example, an impurity that was later identified as the mAb with unprocessed heavy chain leader sequence was insufficiently cleared during the CEX step. For removal of this impurity, operating conditions needed to be modified to incorporate lower loading than normally established within platform and to include a salt gradient elution methodology. Figure 3 shows the elution and pool cutting strategy for removal of this impurity. With these modifications, 90% removal of the impurity could be achieved.
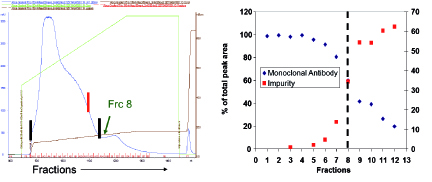
Figure 3: Removal of impurity via modifications of CEX platform operating conditions.
Use of weak partitioning chromatography
Using weak partitioning chromatography with AEX as the only polishing step has also been proposed as a possible efficient platform (5, 6). Compared to more typical flow-through chromatography, here the conductivity and pH are chosen such that the binding of both the product and impurities are enhanced, attaining an antibody partition coefficient (Kp) between 0.1-20, and preferably between 1 and 3. While both the antibody and impurities bind to the anion exchange resin, the impurities are much more tightly bound than in flow-through mode resulting in significantly higher impurity clearance. Load capacity (>1500 g/L) may be achievable with > 95% product recovery and robust process performance.
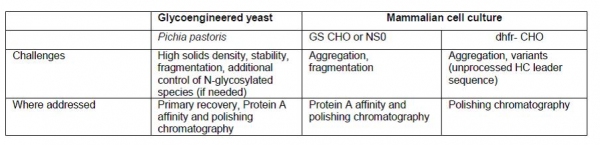
While mammalian cells (e.g., such as Chinese hamster ovary [CHO]), have been the primary expression system for mAb products; other expression systems such as yeast have also been proposed. Table I summarizes some of the process development challenges with mAb quality from the various expression systems and the tools and unit operations options for addressing these challenges.
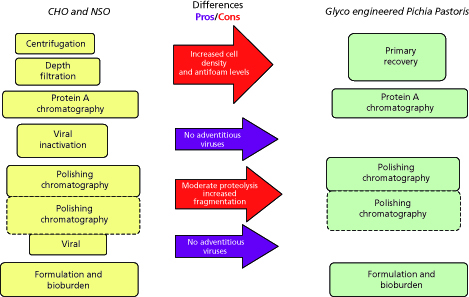
Figure 4: Comparison of purification platform utilized for mAbs expressed in CHO/NS0 and glycoengineered Pichia pastoris.
Further, Figure 4 shows the application of a modular approach for selecting the right modules in the case of purifying mAbs expressed in glycoengineered Pichia pastoris. As shown below, while the primary recovery module is kept as part of the flow scheme, it requires optimization to work with increased solids density, media components, and other metabolites. On the other hand, because of the absence of adventitious viruses in glycoengineered Pichia pastoris, viral inactivation and viral filtration steps, which are part of mammalian cell culture-derived mAb processes, are no longer needed. Primary recovery and initial capture conditions are also optimized to minimize any potential proteolysis from the Pichia pastoris host.
Use of multimodal chromatography
Recently, mixed-mode, ion-exchange ligands with enhanced binding strength for aggregates through the addition of hydrophobic functionality have been brought to market. These resins combine different types of interactions such as ionic interaction, hydrogen bonding, and hydrophobic interaction. This can result in a different selectivity than what is offered by traditional ion-exchange or hydrophobic interaction chromatography, thus offering a larger range of ionic strength and pH under which significant separation can occur (6,7).
Mixed-mode resins based on hydroxyapatite, made from calcium phosphate, have both positive and negative charges and interact with proteins through a combination of electrostatic interactions and coordination complex formation. Traditionally, hydroxyapatite has been used for separation of protein therapeutics from host and media proteins, aggregates, DNA, and Protein A, all of which tend to bind more tightly.
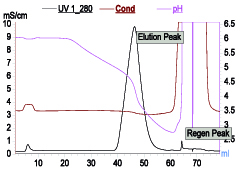
Figure 5A: Elution profile of a mAb on mixed model resin MEP Hypercel. Binding with 25mM citrate pH 6.0. Elution at constant conductivity and pH gradient (pH 6-3). Aggregates: 3% in load 3% and <1% in elution pool.
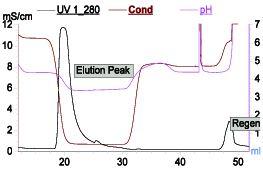
Mixed-mode resins based on ion exchange along with multimodal interactions can offer single-step operation as an alternative to two-step process of ion exchange and HIC. One such example is Capto Adhere (Cytiva, Uppsala, Sweden). It can be operated as anion exchanger as well as hydrophobic mode by applying suitable process condition. In another example MEPHyperCel (Pall Corp., East Hills, NY) resin operates via mixed-mode interactions governed by hydrophobic charge-induction chromatography (HCIC). HCIC is based on pH-dependent behavior of ionizable dual-mode ligand. Binding of protein is based on mild hydrophobic interaction and conducted at near-neutral pH condition where the pyridine group of the ligand is uncharged. Protein desorption is prompted by electrostatic charge repulsion by decreasing the pH. This desorption can be further enhanced by decreasing hydrophobicity. As in HCIC, protein binding can take place at relatively less salt concentration, thus offering an alternative to HIC where high salt requirement for binding is a cumbersome operation. One such profile is shown in Figure 5. It can be operated entirely based on pH or salt with slight change in pH. This resin can also be used as a step for capture step.
These resins offer a different selectivity than the traditional ion exchange and hydrophobic interaction chromatography and are likely to make inroads into many process platforms over time as users create new applications around them.
Alternate capture step
Although Protein A chromatography is a dominant capture step employed in the mAb platform process, it has several major drawbacks. Apart from the relative high cost of the resin, it has limited binding capacity and issues with clean-in-place (CIP) with sodium hydroxide. The alternative can be cation exchangers or hydrophobic charge induction chromatography (HCIC) as a capture step.
Various authors have described cation-exchange chromatography as a capture step in mAb purification (8-11). Though it may not be as efficient as Protein A in terms of selectivity of HCP, it can be used in combination with effective HCP precipitation step. Additionally, to gain similar HCP clearance compared to Protein A, it can be used along with anion exchangers followed by HIC. With the evolution of mixed-mode resins such as Capto Adhere (Cytiva), Ceramic hydroxyapatite (Bio-Rad Laboratories), HEA HyperCel (Pall Life Sciences), and MEP HyperCel (Pall Life Sciences), selectivity can be further enhanced to match Protein A resin (12). One direct advantage of not using Protein A resin is getting rid of leached Protein A and increased life cycle of the capture step. Recent developments have led to availability of cation exchange media with dynamic binding capacities of >100 grams of protein per liter of resin (8). Several commercial processes have been successfully developed using this approach (2).
One major disadvantage of cation exchange as a capture step is the need to precondition the harvest at lower ionic content to promote binding. Though some of the new generation cation exchangers offer high salt binding at pH range of 5 to 6 (11) an alternate is use of HCIC as a capture step (8). This helps in eliminating preconditioning of load from cell-culture harvest. The product can be eluted at lower pH similar to Protein A. Currently, it offers relatively lower binding capacity than Protein A resin but can be a viable option considering the lower cost, high reuse cycle, and lower process development cost in comparison to cation exchange as capture step.
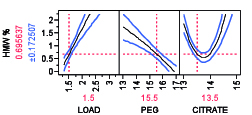
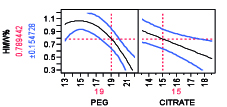
Figure 6A: HMW removal profile as a function of HMW load%, PEG and citrate weight % for a mAb A.
Use of non-chromatographic unit operations
Non-chromatographic unit operations, such as the aqueous two-phase separation, have been long proposed and investigated as an alternative to process chromatography for the purification of mAb products due to its cost effectiveness, high capacity, biocompatibility, and scale up potential (13). Aqueous two-phase extractions (ATPS) that have been proposed for production of mAb products including PEG-phosphate, PEG-citrate, PEG-dextran, and PEG-dextran (14,15). ATPS has been shown to be effective for reduction of low molecular weight (LMW) and high molecular weight (HMW) mAb aggregates (16). Removal of HMW as a function of PEG-citrate concentration, for example, is shown in Figure 6 for two different mAbs. The profiler shown for the model is constructed using design of experiment with varying PEG and citrate weight percentage. It is possible to remove HMW completely by appropriate mixture percentage of PEG citrate at pH 7.0 though the trend may vary from mAb to mAb. A recent study has compared ATPS to the currently established platforms in terms of costs and environmental impact (16). The authors reported that the ATPS offers considerable advantages in terms of process economics, especially when processing high titer cell-culture supernatants. For a given amount of mAb product manufactured, the ATPS-based process reduced the annual operating cost by 40% when cell-culture supernatants with mAb titers higher than 2.5 g/L are processed.
Membrane Chromatography
Membrane chromatography is another emerging alternative to the traditional packed-bed chromatography (17,18). The key benefits it offers are the elimination of diffusive pores and making the mass transfer of the protein to binding sites convective rather than diffusive. The use of convective transfer results in relatively high operational flow rates. Membranes with a variety of ligands (e.g., affinity, ion exchange, hydrophobic interaction) are available on the market. The most popular application involves use of anion-exchange membrane as a polishing step in the mAb purification platform. Such membranes have been shown to be effective in flow-through mode to remove trace amounts of impurities. In a recently published study, the authors demonstrated the feasibility of using hydrophobic interaction membrane chromatography for removal of aggregates and leached Protein-A for a mAb product (19). Due to higher hydrophobicity, the aggregates were removed by selective adsorption on the membrane. Protein-A was removed as it formed relatively hydrophobic complexes with the mAb. Thus, the mAb product eluted in the flow-through while the impurities remained bound to the membrane and could subsequently be eluted by lowering the salt concentration. With the high throughput that membrane adsorbers provide, this could be a promising addition to the antibody purification platforms.
Summary
It is evident that many alterations to the mAb platform process have been implemented by biopharmaceutical companies or are under consideration. With the advent of biosimilars and the related increased pressure on lowering the cost of production, it is likely that the next decade will see more significant changes in the platform.
References
1. The Development of Therapeutic Monoclonal Antibody Products, Editors: H. L. Levine and G. Jagschies (BioProcess Technology Consultants Inc. and General Electric Company, 2010).
2. A.A. Shukla, B. Hubbard, T. Tressel, S. Guhan, and D. Low, J. Chromatogr. B 848, 28-39 (2007).
3. J. Glynn, T. Hagerty, T. Pabst, G. Annathur, K. Thomas, P. Johnson, N Ramasubramanyan, P Mensah. BioPharm Intern. Supplement (2009).
4. G. Rao et al., 243rd ACS National Meeting, BIOT 83, 2012.
5. B. Kelley et al., Biotech and Bioengg., 101 (3), 2008.
6. H. F. Liu, J. Ma, C. Winter and R. Bayer, mAbs, 2, 480-499 (2010).
7. P. Gagnon, Purification of Monoclonal Antibodies by Mixed-Mode Chromatography, in Process Scale Purification of Antibodies, Ed. U. Gottschalk (John Wiley & Sons, Inc., Hoboken, NJ, 2008).
8. G. M. Ferreira, J. Dembecki, K. Patel, and A. Arunakumari, Biopharm Intern., 20(5) (2007).
9. M. Urmann et al., Bioscience In MAbs, 2 (4) pp. 395-404 (July 2010).
10. S. Andreas, and A. Kiesewetter, J.Chromatogr. B 848.1 151-158 (2007).
11. Lain, Blanca, A. M. Cacciuttolo, and G. Zarbis-Papastoitsis, BioProcess International (2009).
12. J.Chen et al., J.Chromatogr. A, 1217(2), 216-224 (2010).
13. I.F. Ferreira et al., J.Chromatogr. A, 1195(1-2), 94-100 (2008).
14. L. N. Mao et al., Biotechnology Progress, 26(6), 1662-1670
15. P. A. J. Rosa, I. F. Ferreira, A. M. Azevedo, and M. R. Aires-Barros, J.Chromatogr. A, 1217 (16), 2296-2305 (2010).
16. N. Fraud et al., BioProcess Intern., June, 30-35 (2009).
17. P.A.J. Rosa, A.M. Azevedo, S. Sommerfeld, W. Bäcker, M.R. Aires-Barros, Biotechnology Advances, 29, 559-567 (2011).
18. A. S. Rathore and A. Shirke, Preparative Biochemistry and Biotechnology, 41, 398-421 (2011).
19. S. M. Yoo and R. Ghosh, Journal of Membrane Science, 390-391, 263-269 (2012).





