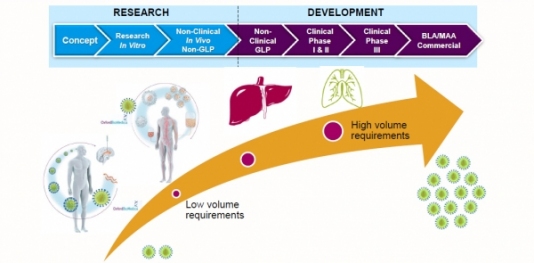
Successes in the clinic have placed many cell and gene therapies on an accelerated route to market. But unless developers consider, at an early stage, how they might produce their product at scale, they may run into problems with commercial manufacturing.
Here, we present an article based on an interview with Carol Knevelman, Vice President, Head of Process R&D at Oxford Biomedica, who shared a case study on large-scale lentiviral vector production at the “Bioprocess Days” event in May 2019. Carol also offers her advice for developing a futureproof commercial process.





