
Viral Clearance Challenges in Bioprocessing Blog Post
Removal and inactivation of adventitious and endogenous viruses have traditionally been achieved…

Removal and inactivation of adventitious and endogenous viruses have traditionally been achieved…

Continued process verification (CPV) is the activity that provides ongoing verification of the…
The quality-by-design principles that enable a manufacturer to limit and control the sources of process variability…
A critical quality attribute (CQA) has been defined as “a physical, chemical, biological…
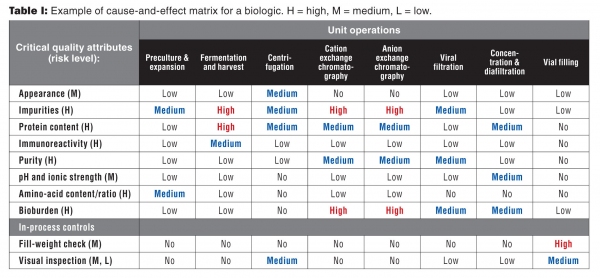
Once risk levels have been assigned to the CQAs, the next activity is to begin to relate which parts…
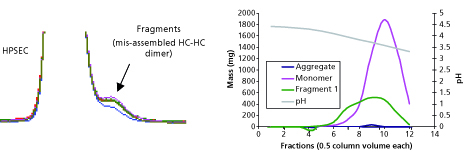

To gain perspective on the implementation of quality by design (QbD) and process analytical…